The molar mass is the magnitude that is most often found when calculating in chemical practice. It denotes a mass of the amount of substance.
In order to calculate the molar mass of the substance, you need to know:
- The formula of the substance molecule, where the number of atoms of the elements included in the molecule is indicated.
- The atomic weight of each of the elements constituting the molecule. It can be found in the table of the periodic system of Mendeleev elements, which has already calculated it for us.
Then we can proceed to the calculation.
I turn out separately by the data of each element, that is, if the atom one, then the standard atomic weight is multiplied by one. If the atoms of the substance in the molecule 2, then, respectively, and multiply atomic weight by 2, and so on.
We summarize the results obtained to obtain a total weight of the molecule.
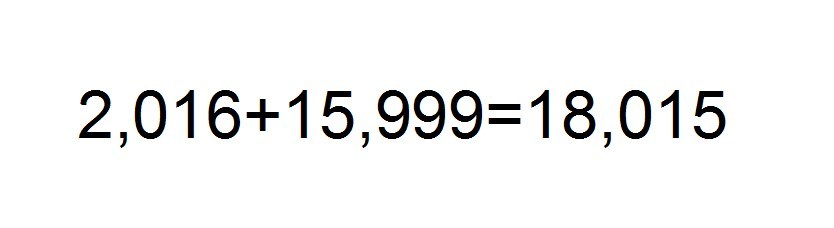
The resulting mass of the molecule is multiplied by 1 g / mol, to produce molar mass. 1 g / mol is a constant of molar mass, and denotes a mass of one praying substance.
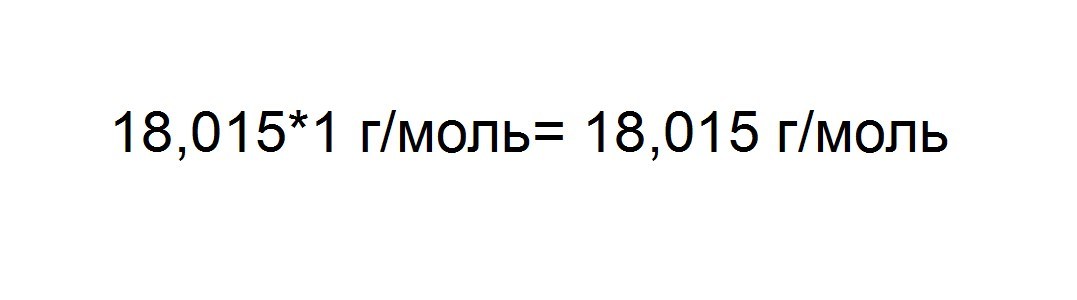
Since the molar mass calculation algorithm is very simple, in the presence of an Internet, for simple formulas, you can use the online calculator.
For more serious formulas, such as CO2h.n: N.CO2H, in order to increase reliability, it is worth making calculations with your own hands, not trusting the developers of calculators.